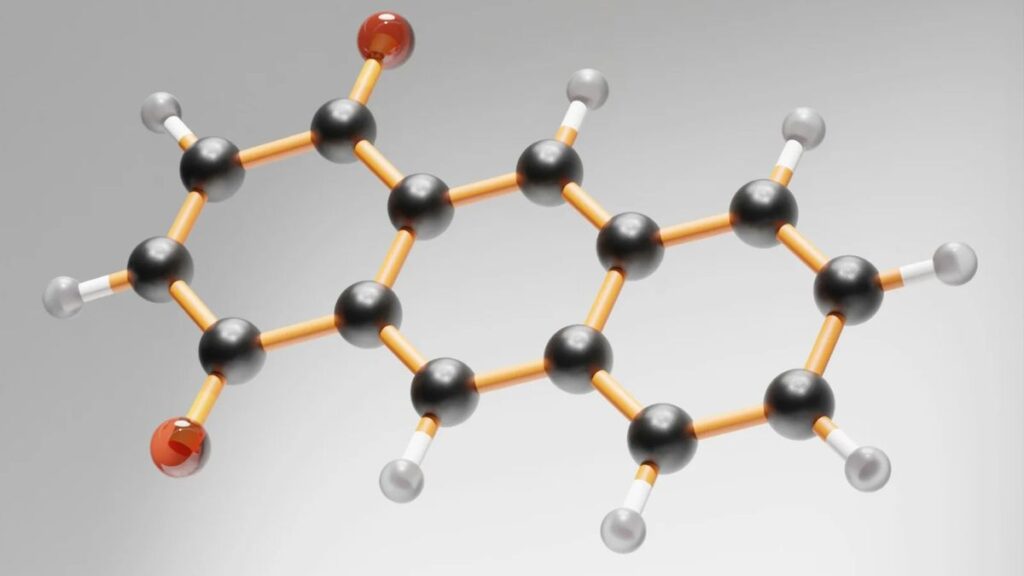
Introduction to the Molecule HCOOCH CH2 H2O
Have you ever wondered about the fascinating world of molecules that make up our everyday lives? One molecule, in particular, has caught the attention of scientists and researchers alike: HCOOCH CH2 H2O. This intriguing compound is more than just a series of atoms; it’s a key player in various chemical processes and applications. From its molecular structure to its unique properties, there’s much to explore about this compound. Join us as we delve into the details of HCOOCH CH2 H2O and uncover what makes it so special!
Molecular Structure and Composition
The molecular structure of HCOOCH CH2 H2O is fascinating. It consists of a formate group (HCOO) connected to a methylene unit (CH2), along with water molecules.
At the core, the carbon atoms form a backbone that gives rise to various functional groups. These contribute to its unique characteristics and reactivity.
The presence of oxygen in both the formate and water sections adds polar qualities. This polarity influences solubility in different solvents, making it versatile for chemical reactions.
Understanding how these components interact allows scientists to predict behavior under various conditions. The precise arrangement also affects stability and potential uses in industries such as pharmaceuticals or agriculture.
Thus, dissecting its molecular composition reveals insights into how HCOOCH CH2 H2O operates at a fundamental level.
Physical and Chemical Properties
HCOOCH CH2 H2O exhibits intriguing physical and chemical properties. This molecule is a colorless liquid at room temperature, making it visually appealing in various applications.
Its boiling point hovers around 100 degrees Celsius, which allows for versatile usage in different environments. The presence of water molecules facilitates hydrogen bonding, enhancing its solubility in polar solvents.
Chemically, HCOOCH CH2 H2O showcases reactivity typical of esters and alcohols. It can participate in condensation reactions and hydrolysis under specific conditions. Its ester functional group contributes to distinctive fragrance characteristics, often used in flavoring agents.
The molecule’s stability under standard conditions makes it suitable for industrial processes. However, exposure to extreme temperatures may lead to degradation or unwanted reactions. These unique attributes add value across numerous domains such as pharmaceuticals and food science while paving the way for innovative research opportunities.
Uses of HCOOCH CH2 H2O
HCOOCH CH2 H2O, also known as methyl formate, has a variety of intriguing applications across different industries. Its use as a solvent is perhaps one of the most notable. This compound effectively dissolves various organic substances, making it valuable in chemical formulations.
In agriculture, methyl formate serves as an effective fumigant. It’s used to control pests and pathogens in stored grains and soil treatments. This usage highlights its role in sustainable agricultural practices.
Moreover, it’s gaining attention in the field of biofuels. Methyl formate can act as a potential fuel or fuel additive due to its favorable combustion properties.
Additionally, researchers are exploring HCOOCH CH2 H2O’s potential in pharmaceuticals for drug delivery systems. Its unique properties could enhance the efficacy of medications by improving solubility and stability.
The versatility of this molecule opens doors for innovative solutions across multiple sectors.
Comparison to Similar Molecules
When comparing HCOOCH CH2 H2O to similar molecules, it’s essential to consider its structural nuances. This compound has a unique ester linkage, which sets it apart from simple alcohols and acids.
For instance, acetic acid (CH3COOH) shares some characteristics but lacks the additional ether functionality found in HCOOCH CH2 H2O. This inclusion enhances its solubility in various solvents.
Another relevant molecule is methanol (CH3OH). While methanol is a straightforward alcohol with different reactivity patterns, HCOOCH CH2 H2O showcases more complex interactions due to its dual functional groups.
Furthermore, comparing it with ethylene glycol reveals distinct differences as well. Ethylene glycol’s diol structure offers unique properties suitable for antifreeze applications that are not applicable to our target molecule.
These comparisons highlight how variations in molecular structure can lead to diverse behaviors and uses across chemical compounds.
Potential Applications and Future Research
The versatility of HCOOCH CH2 H2O opens doors to numerous applications across various fields. Its unique molecular structure makes it a candidate for use in pharmaceuticals, where it may serve as an intermediate or active ingredient.
In agriculture, this molecule could enhance the efficacy of certain pesticides or fertilizers. Researchers are exploring its potential as a biodegradable solvent that minimizes environmental impact while maximizing performance.
Future studies might focus on refining synthesis methods to improve yield and purity. Innovations in material science could lead to new polymers based on HCOOCH CH2 H2O, offering improved durability and functionality.
Exploring its interactions with other compounds can unveil yet unexplored properties and uses. Collaborative research efforts will be key to unlocking its full potential in industry applications and technological advancements.
Conclusion
The exploration of HCOOCH CH2 H2O reveals a fascinating interplay of molecular structure and properties. Understanding this molecule opens doors to various applications, from industrial uses to potential advancements in scientific research. Its unique characteristics distinguish it from similar compounds, showcasing the complexity and diversity found within organic chemistry.
As we delve deeper into its properties, researchers are likely to uncover even more potential that could benefit multiple fields. The ongoing study of such molecules is crucial for innovation and development in both existing and emerging technologies. Each discovery brings us one step closer to harnessing the full power of compounds like HCOOCH CH2 H2O for practical use.
This journey through the world of molecular structures emphasizes how much there is still left to learn about chemical interactions and their implications on our daily lives. With future studies on the horizon, excitement continues to build around what else might be achieved with this intriguing compound.